Uncovering the cellular secrets of RNA editing
By Rob Clancy, staff writer. Reviewed by Professor Carl Walkley
Our cells need to be able to recognise potential invaders as a threat, the key function of the body’s innate immune system. Sometimes this process fails causing the body to react to its own cells leading to autoinflammatory disease. New research has identified a critical fall-back mechanism used by the cells when the usual RNA editing process is switched off.
Professor Carl Walkley heads the RNA Biology and Innate Immune Sensing Research Group at Hudson Institute of Medical Research and their latest research, published in Science Immunology, sheds new light on these crucial processes.
He explained that cells must be able to determine between their own RNA (“self”) and foreign RNA (“non-self”) that may be a threat, such as a virus.
“We know cells use the protein ADAR1 and a process called A-to-I RNA editing as an important way to mark “self” from “non-self” RNA. We have now found a new pathway allowing cells to tolerate losing ADAR1 mediated A-to-I editing, preventing immune reactions from being triggered,” he said.
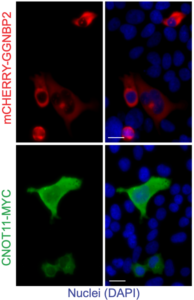
RNA editing identifies invaders
Working with collaborators from the Peter MacCallum Cancer Centre, Victorian Comprehensive Cancer Centre, Spain’s Universidade de Santiago de Compostela, and Stanford University, USA, the team expanded the understanding of how cells can adapt to changes in A-to-I editing and also identifies new ways RNA can be handled in the cell,” he said.
Among the benefits of this new understanding is potential application in Aicardi-Goutieres Syndrome (AGS), a rare inherited disease affecting the immune system and associated with elevated type I interferon, for which there are currently no effective treatments.
Prof Walkley said that ADAR1 mutations are one of the causes of AGS: “Our work identifies previously unknown pathways that can allow cells to tolerate a loss of ADAR1 activity. These new pathways or proteins may be new targets to explore as potential treatments of this disease.”
The multinational research also developed a new cell line model to study loss of ADAR1 functions, as well as isolating key regulators of the autoinflammatory response to unedited self dsRNA and identifying a new pathway regulating the cellular response to immunogenic self dsRNA.
Innate immunity explained
The innate immune system is your body’s first line of defence against harmful invaders like bacteria and viruses. It responds quickly and doesn’t need prior exposure to recognise threats.
It incorporates physical barriers, such as skin and mucous, cells like macrophages and neutrophils that “eat” germs, and chemical signals like cytokines that trigger inflammation. It also includes specialised proteins in the cell cytoplasm that sense and act to destroy invaders.
The innate immune system acts as a rapid response team, buying time for the adaptive immune system to kick in if needed.
A-to-I RNA editing is a process that directly charges the sequence and structure of RNA. It occurs on the cell’s own RNA and we now appreciate that this is an important way that cells can recognise potential RNA threats.
Collaborators | Prof Kaylene Simpson and Dr Iva Nikolic @ Victorian Centre for Functional Genomics, Peter MacCallum Cancer Centre, Victorian Comprehensive Cancer Centre. Dr Adriana Escudero, A/Prof Miguel Fidalgo and Dr Diana Guallar, Center for Research in Molecular Medicine and Chronic Diseases (CiMUS). Universidade de Santiago de Compostela, Spain. Prof Billy Li and colleagues, Department of Genetics, Stanford University, USA
This research was supported by | National Health and Medical Research Council Australia (NHMRC; GNT1183553 (CRW and JHF); GNT1182453 (JHF and AMC); GNT2020743 (JHF and AMC); GNT2018098 (CRW)), 5point Foundation (JHF); Flack Foundation (JHF); SVI Rising Star (JHF). The work was supported in part by the Victorian State Government Operational Infrastructure Support Scheme to St Vincent’s Institute. The Victorian Centre for Functional Genomics is funded by the Australian Cancer Research Foundation (ACRF), Phenomics Australia, through funding from the Australian Government’s National Collaborative Research Infrastructure Strategy (NCRIS) program, the Peter MacCallum Cancer Centre Foundation and the University of Melbourne Collaborative Research Infrastructure Program.
Journal | Science Immunology
Title | GGNBP2 regulates MDA5 sensing triggered by self doublestranded RNA following loss of ADAR1 editing
View publication | https://www.science.org/doi/10.1126/sciimmunol.adk0412
In this article
About Hudson Institute
Hudson Institute’ s research programs deliver in three areas of medical need – inflammation, cancer, women’s and newborn health. More
Hudson News
Get the inside view on discoveries and patient stories
“Thank you Hudson Institute researchers. Your work brings such hope to all women with ovarian cancer knowing that potentially women in the future won't have to go through what we have!”








