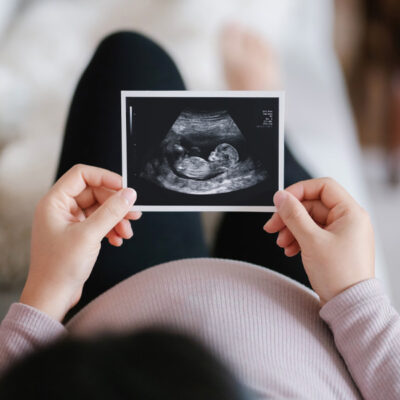
PROTECT Me Trial | Ronick’s story
By Rob Clancy, staff writer
Professor Suzanne Miller and her team’s PROTECT Me Trial is providing hope to parents of our most vulnerable babies like Ronick.

A leader in the field of fetal and neonatal physiology and brain injury, Prof Miller is working to prevent and treat the neurodevelopmental disorders that can have lifelong effects.
“The developing brain is fascinating. The fetal brain is exquisitely sensitive to the in-utero environment. We are still learning how compromise during pregnancy affects the baby’s brain development and long-term neurological outcomes.” Professor Suzanne Miller
World-first PROTECT Me Trial
One of her projects is the world-first PROTECT Me Trial, using melatonin to combat the effects of fetal growth restriction (FGR) – a major cause of cardiovascular disease, lung and brain injury in the newborn – as well as contributing to the ongoing development of cerebral palsy, autism, learning and other behavioural dysfunctions.
“My research goal is to see fewer children with cerebral palsy or learning disabilities because of new preventions and treatments my team has developed for neonatal brain injury.”
Ronick’s story
When doctors told Hannah after her 28-week scan that her baby’s growth was well behind schedule, she was understandably concerned – especially in light of previous miscarriages.

So, when staff at her Monash Health obstetric check-up asked if she would like to take part in the PROTECT Me Trial, she and husband Nav did not hesitate.
“I believe in science. I trust the doctors and midwives, so I decided to join the trial as soon as I knew there was an issue with my baby’s growth,” she said.
Every week, she returned for a scan with an instruction to “pack your bags because you may need an emergency C-section at any time”.
Baby Ronick waited another seven weeks to come into the world. He weighed 1.88kg (small, but still much more than expected) and gave a good healthy cry when he arrived.
“We were so relieved to have a healthy baby!” Hannah said.
Ronick’s progress will be monitored, like the other 335 babies in the trial, and once they have reached their second birthday, the effectiveness of melatonin treatment will be assessed.
Hannah and Nav are proud to have played their part in advancing medical science.
Collaborators | Monash Health; Monash University
Funders | Cerebral Palsy Alliance; Inner Wheel Australia; NHMRC
Contact us
Hudson Institute communications
t: + 61 3 8572 2761
e: communications@hudson.org.au
In this article
About Hudson Institute
Hudson Institute’ s research programs deliver in three areas of medical need – inflammation, cancer, women’s and newborn health. More
Hudson News
Get the inside view on discoveries and patient stories
“Thank you Hudson Institute researchers. Your work brings such hope to all women with ovarian cancer knowing that potentially women in the future won't have to go through what we have!”